Industrial-Scale Seawater Splitting-Green Hydrogen
Industrial-Scale Seawater Splitting-Green Hydrogen
Strategies for Industrial-Scale Hydrogen Production via Direct Seawater Splitting with Long-Term Durability
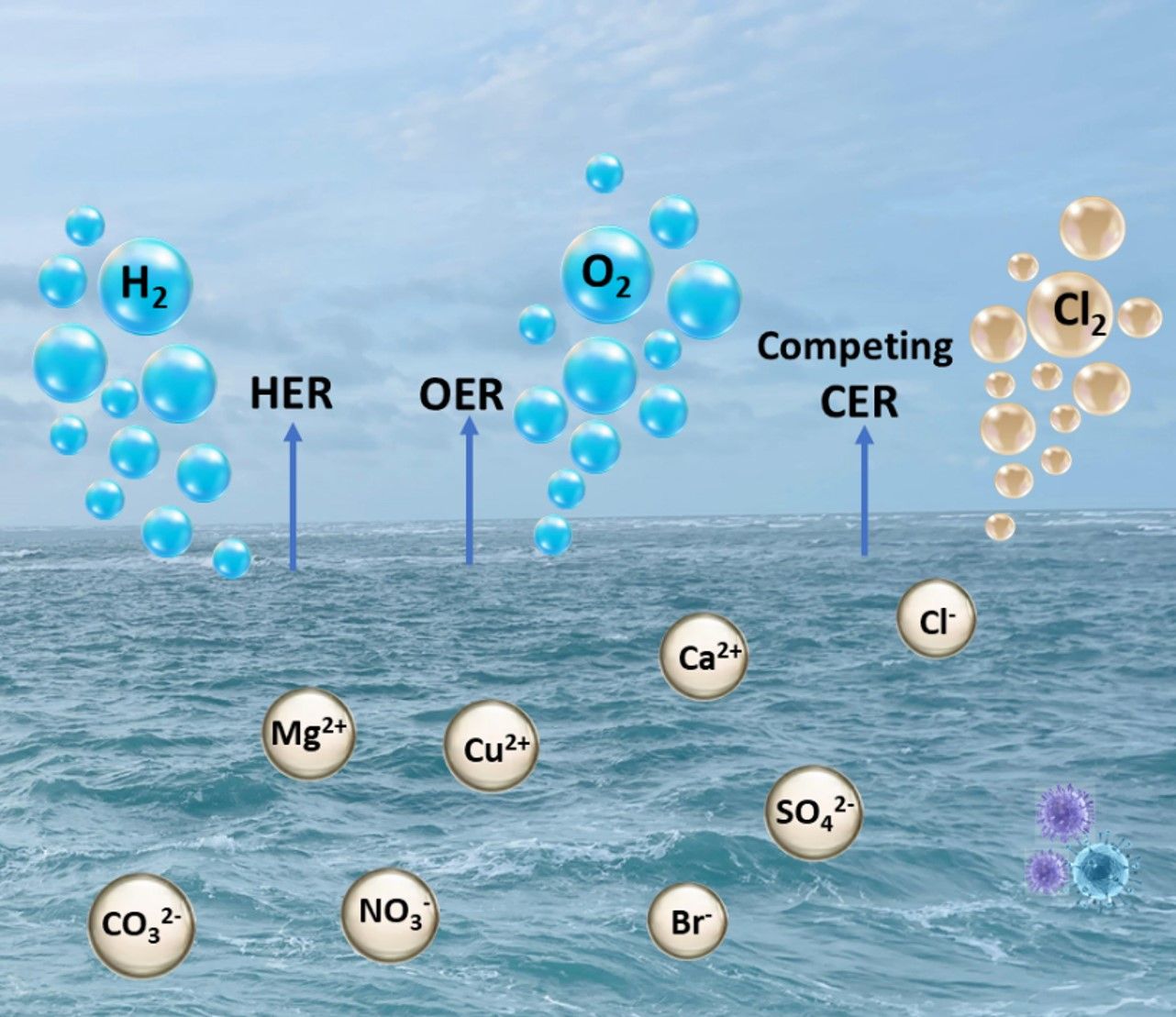
Seawater splitting (SWS) for H2 production using renewable energy sources is a promising approach towards fulfilling green energy needs of current times with easily storable and transportable forms of energy. Similar to fresh water splitting, SWS also consist of mainly two half reactions: O2 evolution reaction (OER) in the anode and the Hydrogen evolution reaction (HER) in the cathode. Despite substantial advancements, large-scale application of SWS is yet not realized due to two major concerns: low catalytic activity and poor long-term operational stability. SWS has two approaches: indirect SWS, where extensive water purification is involved before electrolysis and direct SWS. The indirect SWS electrolyzer requires thorough desalination, which adds to the construction and maintenance costs of the water purification system. Thus, direct SWS is promising for developing efficient and durable electrocatalysts for membrane electrolyzers.
Mechanistic evaluation of catalysts and interfaces is crucial for the industrialization of direct electrochemical seawater splitting for green hydrogen production. The primary challenges associated with direct seawater electrolysis in anode are: (i) Cl- corrosion and (ii) chlorine evolution reaction (CER); and in cathode are: (i) Cl- corrosion, (ii) Ca2+, Mg2+ precipitation, and (iii) metal ion reduction and electrodeposition. This paper addresses rational strategies to mitigate these challenges.
Strategies for preventing Cl- corrosion and CER inhibition
The difference in Gibbs free energy for *Cl and *OH makes it possible for OH- to selectively adsorb over Cl- on the catalyst surface to make M-OOH species. This can push towards OER. Mixed metal phosphide and noble metal-doped transition metal oxide electrocatalysts can provide selective OH- adsorption sites for SWS. Using the hard soft acid base principle, OH- can be preferentially captured by the introduction of hard Lewis acid layer over the electrocatalyst. GuO et al. introduced Cr2O3 hard acid layer over CoOx electrocatalyst for superior OH- capture for natural SWS with good stability for 100 h at 500 mA cm-2. Inducing a passivating anion-rich layer on the electrocatalyst during OER can repel Cl- in seawater. Anions like SO42-, CO32-, PO43-, NO3-, BO2- and SeOx- have shown noticeable inhibition towards Cl- corrosion of the anode in SWS.
The inertness of Ti and Ru in saline water is useful in rational design of electrocatalysts for long-term stability. TiO2 and RuO2 based as well as Ti/Ru doped electrocatalyst show long-term durability in seawater owing to their intrinsic anti-corrosion ability. The high stability and inertness of metal-nitrogen bonds give structural and chemical stability to metal nitrides. Ren et al. reported NiMoN@NiFeN over nickel foam for alkaline SWS, where it achieved a current density of 1 A cm-2 which is industrially required at a low voltage of 1.709 V. Using Ti mesh/plate as substrate adds intrinsic stability to the electrode in Cl- rich mediums where nickel foam gets noticeably corroded after long-term operation.
Another approach to block Cl- penetration is to add a catalytically inert protective layer which allows selective transfer of OH-. Koper el al. demonstrated a porous MnOx layer over IrOx facilitates the transfer of H2O, H+ and O2 while preventing Cl-. However, its lack of stability in low pH, high current density, and dissolution of Mn ions in flow cells limits the use of MnOx in large scale. Ultrathin layers of SiOx, CeOx, PbOx and graphene-based materials have also been used to prevent Cl- penetration for selective OER.
Another contradictory strategy is to selectively adsorb Cl- to improve anode stability for SWS. Xu et al. immobilized Cl- by formation of insoluble AgCl using optimized Ag nanoparticle-decorated NiFe-LDH for long-term durability in 1 M NaOH + seawater for 2500 h at 400 mA cm-2. Duan and coworkers reported tailoring Cl- adsorption sites using the hard-soft acid base principle with Ir-CoFe-LDH electrocatalyst. Here Fe3+ is a softer Lewis acid that prefers to bind with Cl- which is a softer base than OH-. This protects Fe sites from Fe-OH formation, which promotes long-term operational stability. This particular catalyst also benefits from strong Ir-Cl coordination, which stabilizes the Ir single atom sites for SWS.
Strategies to prevent Ca and Mg hydroxide precipitation
Ca and Mg hydroxide precipitation at the cathode during SWS is a major concern; however, only a handful of studies have addressed this problem. The addition of Cr2O3 hard acid layer over CoOx electrocatalyst for OH- capture can lower Ca and Mg hydroxide precipitation in neutral or near-neutral pH seawater. Using the abundance of Mg2+ in seawater, nanoscale Mg(OH)2 can be synthesized, as demonstrated by Lu and co-workers. They have developed a solidophobic surface of NiCu alloy over Pt for enhanced H2 production as well as Mg(OH)2 production with high purity. The solidophobic electrode surface ensures homogeneous nucleation of Mg(OH)2 in the electrolyte. This approach requires the design of electrolyzer for steady Mg(OH)2 collection, for which Liang et al. have proposed an electrolyzer design incorporating linear flow channels and regular acid flow to maintain the electrolyte pH. Physical methods such as spatially designed 3D electrode substrates for generation of uniform bubbles and disrupting OH- gradient with external water flow and removing large precipitates near the cathode are attractive for industrial SWS. Similar strategies can be used to prevent the electrodeposition of different metals like Cu or Pb on the electrode surface.
Conclusion and prospects
Renewable energy-powered electrochemical SWS for green H2 production can enable direct use of seawater without extensive water pretreatment, which reduces the complexity of the electrolyzer as well as increasing efficiency. Curranty researchers worldwide are focusing in this direction however, to achieve industrial applicability, the following aspects are to be taken into account:
(a) Use standardized test conditions to mimic natural seawater, as basic NaCl electrolyte is not analogous for seawater
Other than the presence of Br-, SO42-, CO32-, NO3-, microbial contaminants and solid impurities can interfere in reaction kinetics and also lead to electrode decay.
(b) Development of advanced functional membranes is essential for efficient large-scale electrolyzers that can operate on natural seawater without the need of extensive pretreatment or purification
Based on the present literature, an AEM with high OH- conductivity and good mechanical/chemical stability in low-grade water is required for operation. Similarly, membranes for ultra/nano filtration of natural seawater are also crucial before feeding into SWS cell or stacks.
(c) For industrial applications, scalable electrode substrates and electrolyzer design are also crucial, as is scalable synthesis of electrocatalysts
Larger SWS cells and stacks need to overcome challenges faced by PEMWE and AEMWE today. Design of a novel electrolyzer with linear flow channels to remove precipitate buildup and steady collection of electrosynthesized Mg(OH)2 needs to be focused. Today, direct SWS technology is far from industrial applicability; however, the scale and applicability of SWS systems should reach industrial scale H2 production levels over the coming decade. Again, to alleviate pressure in the clean water reserve, the advances in SWS can be expanded to other low-grade water sources like sewage or industrial waste water.
References & Further Reading
1. W. He, X. Li, C. Tang, S. Zhou, X. Lu, W. Li, X. Li, X. Zeng, P. Dong, Y. Zhang, Q. Zhang, ACS Nano, 2023, 17, 22227−22239. https://pubs.acs.org/doi/10.1021/acsaem.4c01290
2. Y. Liu, Y. Wang, P. Fornasiero, G. Tian, P. Strasser, X. Y. Yang, Angew. Chem. Int. Ed. 2024, 202412087. https://onlinelibrary.wiley.com/doi/abs/10.1002/an...
3. J. X. Guo, Y. Zheng, Z. P. Hu, C. Y. Zheng, J. Mao, K. Du, M. Jaroniec, S. Z. Qiao, T. Ling, Nat. Energy. 2023, 8, 264–272. https://www.nature.com/articles/s41560-023-01195-x
4. L. Yu, Q. Zhu, S. W. Song, B. McElhenny, D. Z. Wang, C. Z. Wu, Z. J. Qin, J. M. Bao, Y. Yu, S. Chen, Z. F. Ren, Nat.Commun. 2019, 10, 5106. https://www.nature.com/articles/s41467-019-13092-7
5. J. G. Vos, T. A. Wezendonk, A. W. Jeremiasse, M. T. M. Koper, J. Am. Chem. Soc. 2018, 140, 10270–10281. https://pubs.acs.org/doi/10.1021/jacs.8b05382
6. W. W. Xu, Z. F. Wang, P. Y. Liu, X. Tang, S. X. Zhang, H. C. Chen, Q. H. Yang, X. Chen, Z. Q. Tian, S. Dai, L. Chen, Z. Y. Lu, Adv. Mater. 2023, 36, 2306062. https://onlinelibrary.wiley.com/doi/abs/10.1002/ad...
7. X. Duan, Q. Sha, P. Li, T. Li, G. Yang, W. Liu, E. Yu, D. Zhou, J. Fang, W. Chen, Y. Chen, L. Zheng, J. Liao, Z. Wang, Y. Li, H. Yang, G. Zhang, Z. Zhuang, S.-F. Hung, C. Jing, J. Luo, L. Bai, J. Dong, H. Xiao, W. Liu, Y. Kuang, B. Liu, X. Sun, Nat. Commun. 2024, 15, 1973. https://www.nature.com/articles/s41467-024-46140-y