New Era of Green Hydrogen: Anion-Exchange Membrane Electrolyzers
New Era of Green Hydrogen: Anion-Exchange Membrane Electrolyzers
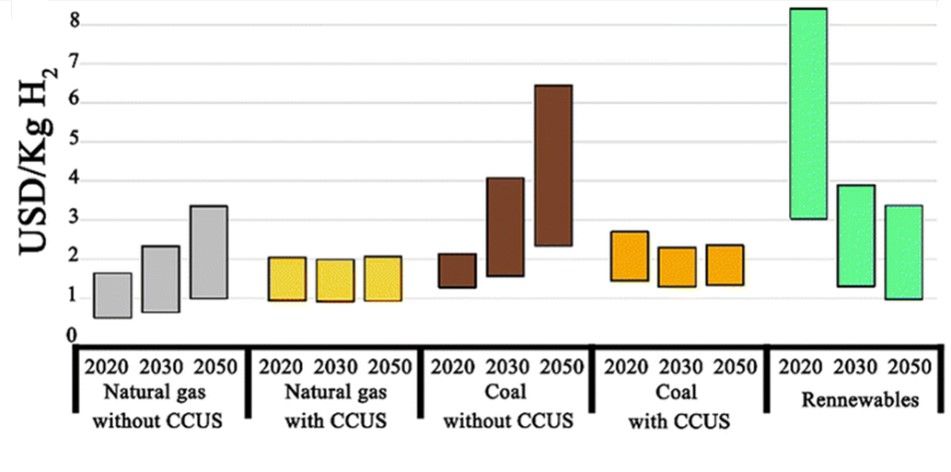
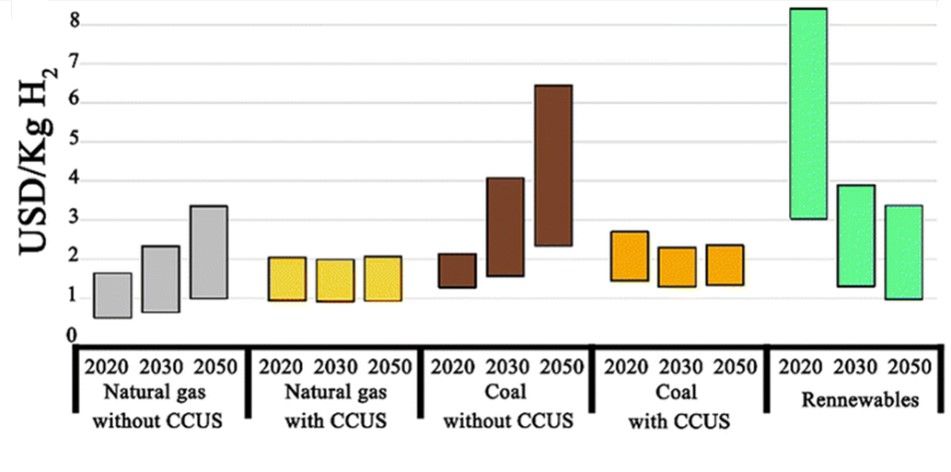
The onset of the industrial revolution led to the development of various industrial sectors like transportation, petrochemicals, and metallurgy, which were and are still powered by fossil fuels. The burning of these fossil fuels leads to the emission of greenhouse gases, eventually harming both the health and environment. It is reported that the CO2 concentration is approaching 420 ppm, which is 50 % higher than pre-industrial revolution era. The total worldwide energy consumption reached 15 TW in 2008 and is expected to double by 2050 and triple by the end of 21st century. As such, to meet the growing energy demands while also focusing on environmental concerns, extensive research needs to be done on the development of alternative clean sources of energy, i.e., those produced using zero-carbon renewable energy resources like wind, solar, hydropower, or geothermal. However, due to the intermittent and fluctuating nature of these sources, energy storage or conversion to chemical fuels is an essential part of harvesting energy as and when needed. Widespread use of H2 can reduce our dependence on fossil fuels and help in the decarbonization of various industries, as it is the ideal chemical for storage owing to its high energy density of 120 MJ kg-1. Compared to electrochemical storage, viz. batteries, energy storage in bonds of molecules like hydrogen does not suffer from self-discharge during storage. However, industrially, hydrogen in almost entirely produced from fossil fuels, contributing to 830 million tons of CO2 emissions per year. Industrial hydrogen is predominantly produced through steam methane reforming (SMR) which emits around 5.5 kg of CO2 for the production of 1 kg of H2. The levelized cost of hydrogen (LCOH) produced from fossils ranges from USD 0.5 to 1.7 per kg while those combined with CCUS (carbon capture, utilization, and storage) technologies is up to 2.0 USD in 2020. The LCOH for green hydrogen, however, is around 3–8 USD per kg which restricts its cost effectiveness and wide industrial usage. Various studies indicate that the LCOH for green H2 will fall to 1-3 USD per kg in the near future with an increase in the price for grey H2 due to various carbon taxes levied by various countries to prompt a higher focus on the development of water electrolyzers for green H2 production.
Figure depicts LCOH via different technologies and in the net zero emission scenario for 2030 and 2050. Image credits Royal Society of Chemistry, reproduced with permission from doi.org/10.1039/D2CS00038E.
Alkaline water electrolyzer (AWE) is the most mature and widely used electrolyzer since their development in 1950s. AWEs employ a porous separator and alkaline electrolyte, typically 30 wt% KOH. The advantage of AWE is the possibility to use non platinum group metal (PGM) as electrodes for HER and OER, thereby reducing the overall cost of production. However, the use of corrosive and concentrated electrolytes, low current density, and reduced operating pressure limit their large-scale usage. Proton Exchange Membrane Water Electrolyzers (PEMWE) are another form of electrolyzers that are well developed and widely used industrially. PEMWEs employ a proton exchange membrane for the transfer of protons from anode to cathode and separate the hydrogen and oxygen produced during the reaction. PEMWEs can operate at higher current densities up to 2 A cm-2 and have a lifetime exceeding 10000 h while the kinetics of PEMWEs are quite faster than AWEs due to acidic conditions involved. However, the requirement of acidic conditions for operations restricts the use of only PGMs like Pt, Ir, Ru as electrodes, thereby increasing capital expenditure. Additionally, use of fluorinated membranes like Nafion and Aquivion also leads to environmental concerns.
Anion-exchange membrane water electrolyzers (AEMWEs) indeed offer a unique combination of benefits, leveraging the strengths of both alkaline water electrolyzers (AWEs) and proton-exchange membrane water electrolyzers (PEMWEs). By utilizing a solid polymer electrolyte in the form of an anion-exchange membrane (AEM), these systems can achieve high operational efficiency and flexibility in terms of current densities and pressures, much like PEMWEs. However, AEMWEs have the additional advantage of functioning in an alkaline environment, which is less corrosive than the acidic environments typical in PEMWEs. This reduction in corrosion risk opens up opportunities to use more affordable, non-precious group metal (non-PGM) catalysts. Specifically, low-cost catalysts based on first-row transition metals such as oxides, nitrides, and sulfides can be used in AEMWEs instead of the expensive platinum-group metals (PGMs) typically required in PEMWEs. The performance and durability of anion exchange membranes (AEMs) depend on key factors like ion exchange capacity (IEC), OH⁻ conductivity, water uptake capacity, and chemical and mechanical stability. Polymer backbones like polysulfones, poly(ether ketone), and polybenzimidazole provide structural integrity, while cationic headgroups mainly N-based groups such as ammonium, piperidinium, and imidazolium—facilitate ion transport. Water uptake and swelling must be carefully controlled to balance conductivity and stability. Non-N-based cationic groups, like phosphonium and metal-based systems, are also explored for enhanced performance. A primary challenge in developing anion exchange membranes (AEMs) is preventing the degradation of cationic groups due to hydroxide anion attack, which neutralizes them and reduces conductivity. This degradation can occur through Hofmann elimination, SN2 substitution, or N-ylide formation. To address this, AEMs can be modified to enhance stability and improve H2 production rates, such as by optimizing the structure of the cationic groups, using more stable polymer backbones, or incorporating alternative cationic moieties that are less prone to degradation under alkaline conditions. Research is primarily focused on development of AEMWEs having high OH⁻ conductivity (> 0.1 S/cm) and robust chemical/mechanical stability at current densities > 3 A/cm² and temperatures > 60°C, in alkaline and oxidative environments. Advancements in anion exchange membrane (AEM) technology have driven the commercialization of several high-performance AEMs, such as Fumasep® FAA-3, Sustainion® X37-50, Tokuyama A201 and A901, Aemion™, PiperION®, and Orion TM1, with improvements in fabrication, conductivity, and mechanical and physicochemical stability. Since membrane is the primary component of AEMWEs, much focus is given on the development of low-cost, and high-performance membranes, while long-term stability under practical working conditions (1000 h or longer) are rarely reported. As such, despite all these developments, there exist several concerns that require extensive investigations on both commercially available AEMs and the development of newer ones for use in water electrolyzers such as: (a) setting up standard measurement techniques for comparison of various properties of commercial membranes and those under development, (b) building on successful results from commercial AEM testing, researchers can explore and innovate new AEM types by leveraging existing structures and formulations, advancing performance and stability in electrochemical systems. (c) development of robust PGM-free catalysts compatible with AEMs for reducing the cost of water electrolysis cells. So, testing can prioritize the compatibility of PGM-free catalysts with AEMs and focus on evaluating long-term durability for practical applications. Addressing these concerns will be critical to the future development of cost-effective, high-performance AEMs for widespread use in water electrolysis.
References & Suggested Reading
- Yang, Y., Li, P., Zheng, X., Sun, W., Dou, S.X., Ma, T. and Pan, H., 2022. Anion-exchange membrane water electrolyzers and fuel cells. Chemical society reviews, 51(23), pp.9620-9693.
- Du, N., Roy, C., Peach, R., Turnbull, M., Thiele, S. and Bock, C., 2022. Anion-exchange membrane water electrolyzers. Chemical reviews, 122(13), pp.11830-11895.
- Shaik, S., Kundu, J., Yuan, Y., Chung, W., Han, D., Lee, U., Huang, H. and Choi, S.I., Recent Progress and Perspective in Pure Water‐Fed Anion Exchange Membrane Water Electrolyzers. Advanced Energy Materials, p.2401956.